Diabecell
Living islet cells to restore the intrinsic ability to control blood glucose for people living with Type 1 diabetes
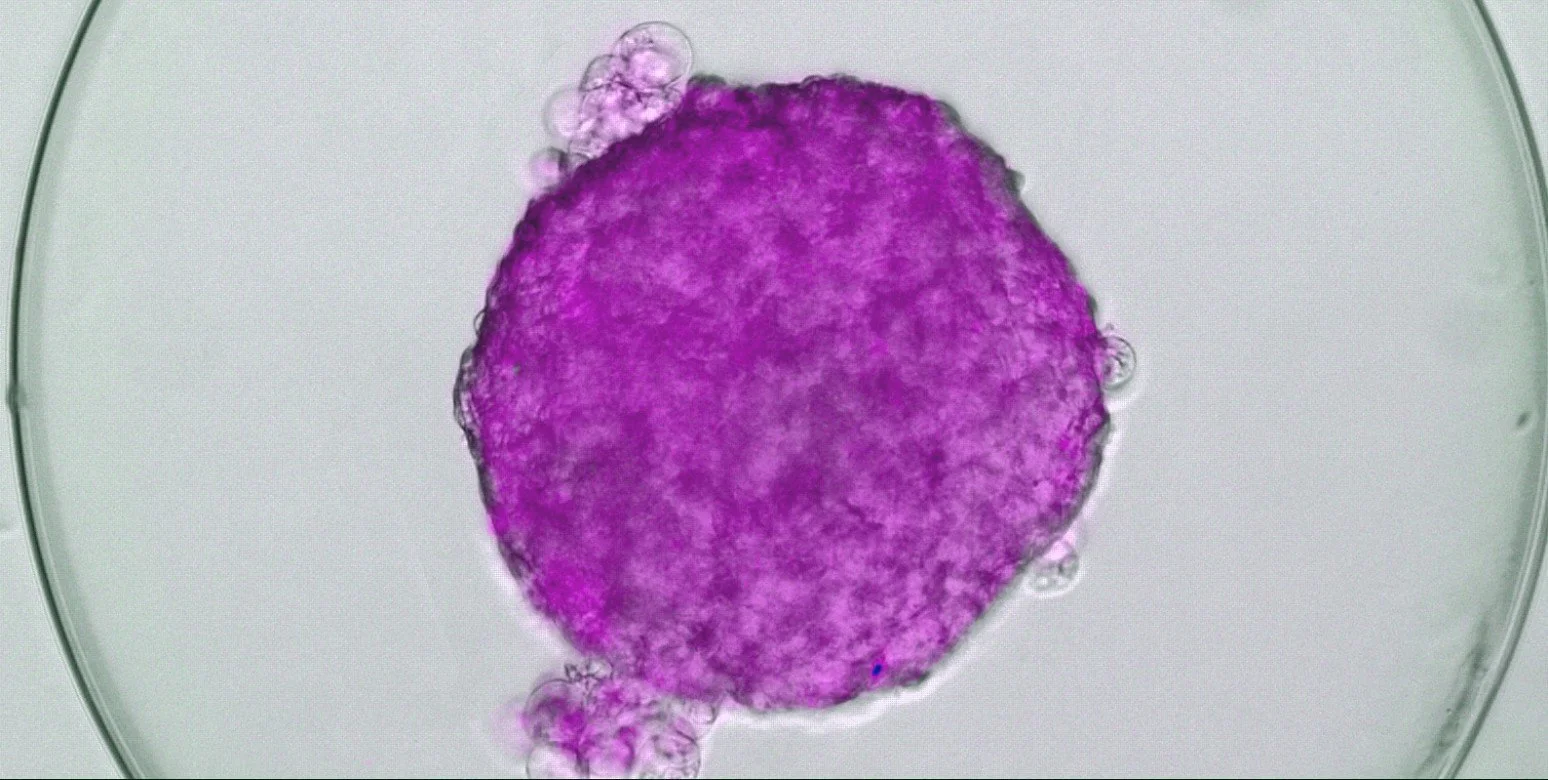
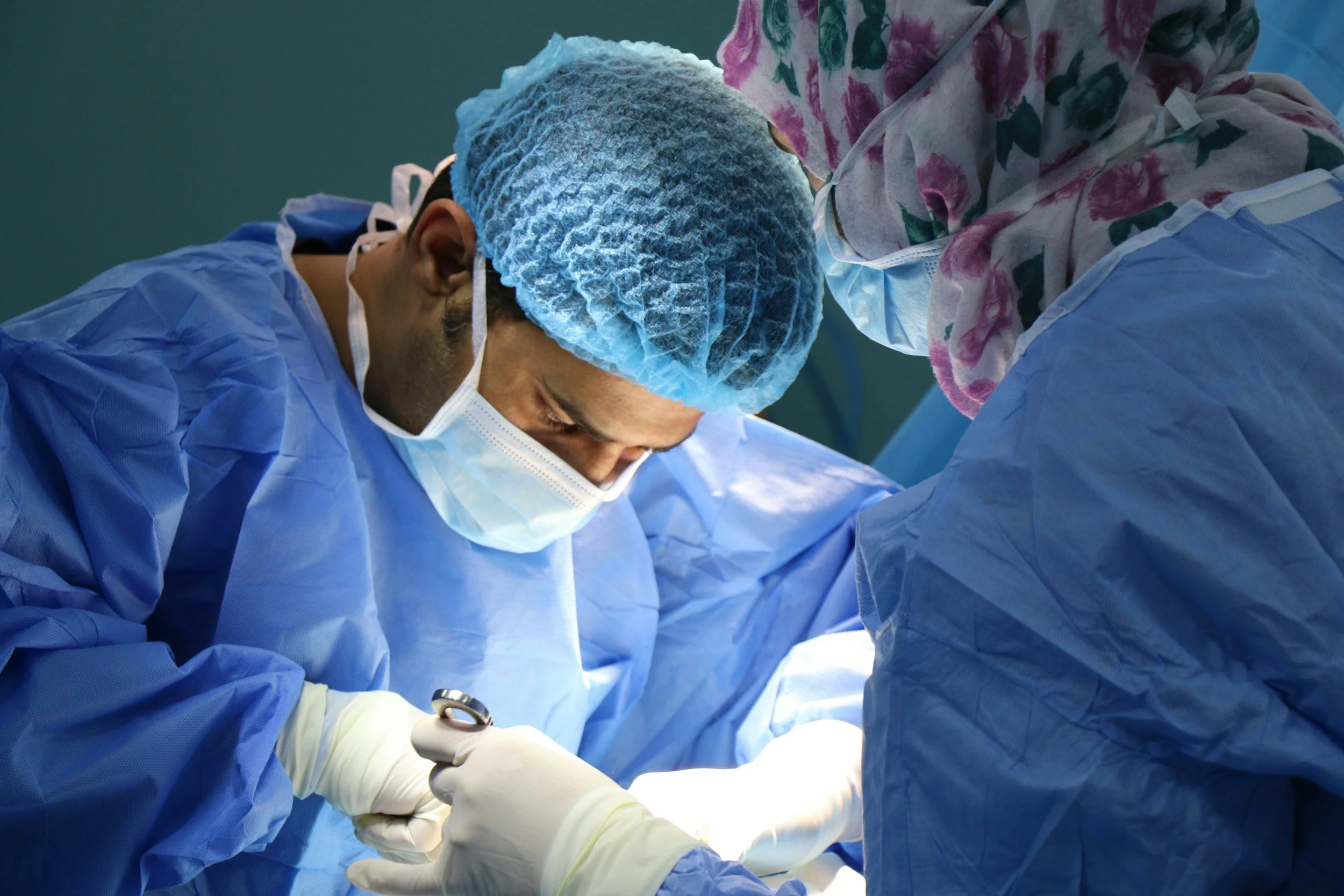
DIABECELL offers a promising solution for Type 1 diabetes by transplanting encapsulated pig islets into patients. This simple surgical procedure replaces lost human islets, restoring the body's natural blood glucose control. Encapsulated in a jelly-like capsule, the pig islets are protected from the patient's immune system. This innovative approach aims to give individuals with type 1 diabetes their independence and improve their quality of life.
DIABECELL has been tested in 38 patients across three international clinical studies to assess its safety, determine the optimal dose, and gain an early understanding of its effectiveness in managing Type 1 diabetes. (NCT00940173, NCT01739829 and NCT01736228). Results indicate DIABECELL can significantly reduce unaware hypoglycemic events and allow patients to lower their insulin dosage without increasing HbA1c levels.
Long term follow-up demonstrated robust microbiological safety and that the majority of patients had positive opinions related to the therapeutic approach 10 years after transplantation.
DOL is providing analytical, molecular diagnostics and xenosafety services to its parent company, Otsuka Pharmaceutical Factory (OPF), in their Phase III study in the U.S. to further evaluate these benefits.
Xenotransplantation
The transplantation or infusion of live cells, tissues or organs from an animal source into a human recipient.
Porcine power
Pig biology is surprisingly similar to human biology. Medicine has long has exploited this similarity to develop biological treatments for disease. Proteins from pigs in particular have been developed and used to great success. Examples include heparin for blood thinning, Factor VI to treat haemophilia and of course, pig insulin to treat type I diabetes.
Type 1 Diabetes
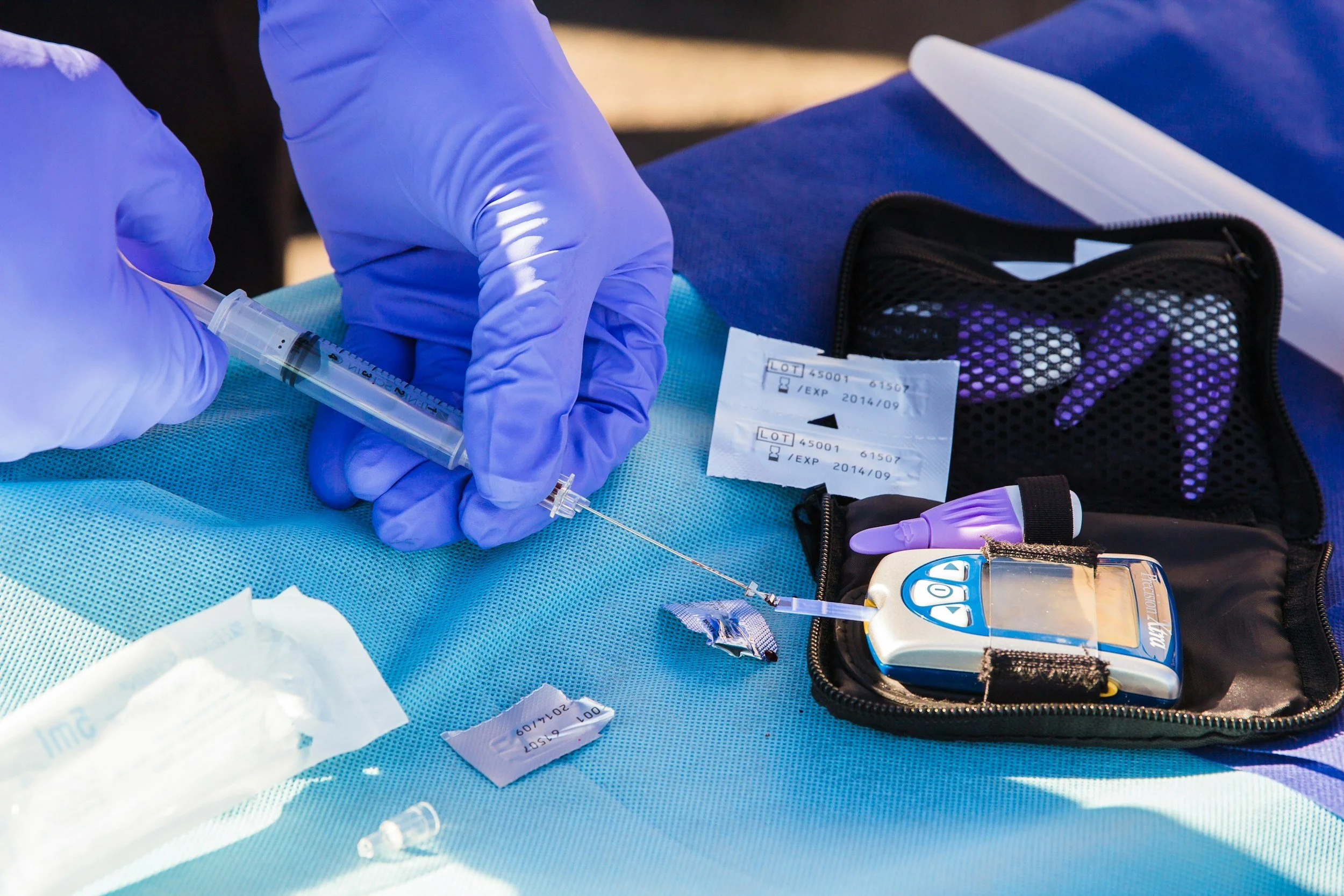
Type 1 diabetes, also known as juvenile or insulin-dependent diabetes mellitus, occurs when the body's immune system destroys the insulin-producing cells (islets) in the pancreas. This prevents the pancreas from producing insulin, which is essential for cells to absorb glucose. The disease typically develops in childhood and requires lifelong insulin injections. Currently, around 8 million adults and 3 million children globally live with Type 1 diabetes.
Hypoglycemia in Type 1 Diabetes
Around 5-15% of individuals with type 1 diabetes (540,000-1,600,000 globally) are highly sensitive to insulin and lack counter-regulatory hormones. This leads to recurrent severe hypoglycemia (low blood glucose). Without warning symptoms like sweating or tremors, individuals may experience dizziness, confusion, blurred vision, and in severe cases, coma, seizures, or death.
The fear of hypoglycemia reduces quality of life and can hinder treatment adherence, impacting glycemic control. Poor control can lead to long-term complications such as damage to the eyes (blindness), kidneys (renal failure), heart (heart disease, stroke), and nerves (foot ulcers, amputation).
DOL, OPF & DIABECELL
In 2018, OPF licensed the rights to develop and commercialise DIABECELL in Japan and USA. At the same time, OPF acquired 100% ownership of DOL and has retained DOL’s international expertise in analytical and molecular diagnostics to establish its manufacturing capability and pig herd in the USA for OPF’s Phase 3 study.
DOL retains the rights to commercialise DIABECELL in the rest of the world and this remains a core part of our long-term strategy, complementing our unique expertise in ensuring the quality and safety of advanced medicinal products across a spectrum of technologies and diseases.